Overview of PET/CT Imaging in Recurrent Prostate Cancer – Current and Emerging Techniques
Over the last few years, we have seen tremendous activity in the area of molecular imaging for prostate cancer. Just about every day we have colleagues asking about the various PET/CT imaging tests - what is available? How do they compare? What are the parameters for successful imaging?
We are proud to have contributed to this body of knowledge. Our work regarding C11-Acetate PET/CT imaging in the recurrent PCa setting with relationship to PSA kinetics has been recently published - representing the largest single-site evaluation of a molecular imaging agent. A link to the publication and brief overview of PET/CT Imaging for Prostate Cancer follow for your review. We hope you find this review useful.
CONVENTIONAL TYPES OF IMAGING
Ultrasound has a role in doing prostate biopsies and the placement of radioactive seeds in primary prostate cancer. It is also useful for evaluating local recurrence after surgery in patients with an increasing PSA. CT Scans are commonly used for staging men with newly-diagnosed disease, looking for enlarged lymph nodes in the pelvis. However, it is inaccurate for detecting cancer in the lymph nodes. If cancer is present in the nodes, a CT scan only finds it 35% of the time. Prostate MRI is used for staging, biopsy guidance, surgical planning, radiation planning, and restaging after PSA relapse. Multi-parametric MRI is being found to be very helpful for detection and local staging of untreated prostate cancer, to reveal features such as extra-capsular extension or seminal vesicle invasion, thus helping to confirm local (organ confined) disease. Additionally, multi-parametric MRI is emerging as a useful imaging tool for following changes in the prostate gland for men on active surveillance.
TECHNETIUM-MDP
Prostate cancer frequently metastasizes to the bone, therefore the mainstay of imaging for advanced prostate cancer has been technetium-labeled (Tc99) bisphosphonate bone scintigraphy. Tc99 bone scans are used for initial staging of intermediate-to-high-risk disease and for restaging after PSA relapse. Unfortunately, it is not sensitive enough to detect small skeletal metastases. False positives are common due to interference from non-cancerous arthritic changes and/or prior trauma.
SODIUM FLUORIDE (NaF) PET/CT SCANS
NaF PET/CT is similar to standard bone scans, but uses PET imaging which is significantly more sensitive and specific than standard Tc99 (Technetium) bone scans. In the PCa recurrent setting, bone lesions as small as 2-3 mm with PSA values < 1.0 can be detected. Unlike Tc99m bone scan, arthritic changes and prior trauma are much less problematic with NaF PET/CT. Another advantage of NaF PET is the shorter scan time, typically less than one hour, compared to 4 hours for Tc99.
11C CHOLINE AND ACETATE PET/CT SCANS
Prostate cancer cells rely on fatty acid metabolism as their energy source. 11C-choline and 11C-acetate are lipid metabolism PET agents and appear useful for detecting recurrent disease after a PSA relapse. 11C-choline has been approved for use at Mayo Clinic while 11C-acetate remains under investigation for this purpose as is not yet FDA approved. Small direct published comparison studies of 11C-acetate and 11C-choline have revealed no clear clinical differences between these agents (Nuklearmedizin. 2003 Feb;42(1):25-30 , Eur J Nucl Med Mol Imaging. 2013 Jul;40 Suppl 1:S18-27)
In a large-scale study of 11C-acetate PET/CT imaging in 887 patients with relapsing PSA (at Phoenix Molecular Imaging), the overall detection rate of recurrent prostate cancer was 88% with a PPV of 91%. A PSA threshold of 1.09 ng/mL was established for optimal imaging. However, if the PSA was less than 1.0 ng/mL and the PSA doubling rate was brisk (less than 3 months), the detection rate was better than 90%. (Am J Nucl Med Mol Imaging 2017;7(1):1-11) The reported detection rate for 11C-choline generally ranges from 42-82% with a PSA threshold of 2.0 recommended for optimal imaging. At least one study in 102 patients has also demonstrated a significant influence of the PSA doubling time on 11C-choline with a 93% detection rate noted in a PSA range of 0.67-1.1 ng/ mL if the PSA doubling rate was under seven months.
11C has a short half-life of 20 minutes, so 11C-Acetate and Choline are available only at sites with cyclotrons capable of producing this agent alongside of the imaging facility. This therefore limits its availability to specialized centers.
C11-Acetate PET/CT in a 66 y/o with Gleason 3+4=7 disease. Prostectomy two years previously and now with a rising PSA of 0.3 ng/mL.
Imaging shows a small metabolic focus in the left prostate bed (top image) and a small positive left external iliac node (lower image). The results of the scan changed treatment plans. Salvage radiation treatment was performed and with the treatment field extended to include the pelvic lymph nodes.
AXUMIN PET/CT SCANNING (18F-FACBC)
Amino acids, such as leucine, methionine, and glutamine, are absorbed into the cancer cells because of the increased metabolic demands of the growing cancer cells. The FDA recently approved Axumin (Fluciclovine or 18F-FACBC), which is a fluorine-18 radiolabeled synthetic leucine amino acid.
CLINICAL TRIALS OF AXUMIN
Scans were performed in 105 patients. The results were checked for accuracy with biopsy or surgery after the scan. Three independent reviewers analyzed the scan results. For men who had biopsy confirmation of cancer in the prostate bed, the true-positive rate ranged from 49-58%. The false positive rates ranged from 16-30%. For patients who had positive biopsies outside of the prostate bed, the results were much better, with a true-positive rate of 88-93% and a false-positive rate of only 7-8%. Optimal detection rates were seen when the PSA was above 1.78 ng/mL.
In another clinical trial of 96 patients, a comparison was made between Axumin and 11C-choline PET. The scans showed equivalent findings 61-77% of the time. However, this study did not include biopsy confirmation. In a third study performed in Italy, 89 patients with a rising PSA were studied. The overall cancer detection rate was only 37%. In those patients with a PSA of less than 1.0 ng/mL, the detection rate was 21%, with a PSA of 1.0-2.0 ng/mL detection was 29%, and when the PSA was higher than 3.0 the detection rate was 59%. %. In a more recent multi-center study of Axumin in 596 patients, the overall detection rate was 67.7% with a true positive rate of 62% and false positive rate of 38%. The mean PSA level was 5.43 ng/mL. For those with a PSA levels <0.79 ng/mL the detection rate was only 41.4% (http:/www.jurology.com/article/S0022-5347(16)31518-X/pdf).
We and others have observed that with Axumin, there is typically much higher muscle and bone marrow background activity than that seen with 11C-Acetate or Choline. This makes small lesions in the prostate bed and bone difficult to detect (see examples below). In some patients, the muscle uptake of Axumin is sufficiently high as to render the study non-diagnostic, despite having properly abstained from physical activity prior to the scan. Additionally, in a small but significant number of patients, interfering urinary excretion is seen. These factors may explain the apparent significantly lower performance of this agent compared to other agents such as PSMA, which are more specific for prostate cancer.
Axumin study showing high physiologic muscle background uptake, rendering the study suboptimal for evaluation of suspected sites of metastasis.
Detection of a bony metastasis. Top row shows an Axumin study in a patient with Gleason 4+4=8 disease, post prostatectomy and salvage radiation two years previously. PSA now rising, 1.1 ng/mL. Fused Axumin PET/CT on the top left and axial Axumin PET on the top right. The Axumin study is negative.
Bottom row of same patient after 11C-Acetate PET/CT imaging clearly shows a metastasis in the body of T5 which was not detected on the Axumin scan. The presence of the T5 lesion was confirmed on a subsequent bone scan and MRI.
PSMA PET/CT SCANS
The prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein that occurs much more commonly in prostate cancer cells compared to benign prostate tissue. The clinically approved imaging method using PSMA was ProstaScint. ProstaScint, however, has several limitations. The technique uses an intact antibody which targets the internal portion of the cell membrane glycoprotein (PSMA) which requires long circulating times. There is prolonged blood-pool retention leading to high background signals, low detection rates, and much lower spatial resolution compared to PET.
Better agents for detecting PSMA have been developed, such as 68gallium-PSMA-11. Several retrospective studies have indicated a higher diagnostic efficiency of 68Ga-PSMA PET/CT compared to 11C-choline PET. In one study, for example, with 319 patients with PSA relapse, an overall 82.8% detection rate was seen. As might be expected, the probability of detecting lesions was correlated with PSA level. A 50% detection was seen when the PSA was 0.2-0.5, 58.3% detection with a PSA of 0.5-1.0, 71.8% detection with a PSA of 1.0-2.0, and 93% detection when the PSA was over 2.0.
AXUMIN VS. 68GALLIUM-PSMA-11
A recently published small pilot retrospective study compared Axumin with 68Gallium-PSMA -11 in a case series of 10 patients with recurrent prostate cancer imaged in short sequence (mediun time of 2.2 months between scans). Five of 10 patients (50%) were negative with Axumin but positive with 68Ga-PSMA-11 PET/CT. The PSMA scan detected a greater number of lymph nodes. Two of 10 patients (20%) were positive with both 18F-fluciclovine and 68Ga-PSMA-11 PET/CT, but 68Ga-PSMA-11 PET/CT showed additional lymph nodes metastasis compared to Axumin. Three of 10 patients (30%) were negative with both 18F-fluciclovine and 68Ga-PSMA-11 PET/CT. While this was a very small study, this translated to a detection rate of 70% for the PSMA agent while Axumin had a detection rate of only 50% with disease underestimated in almost half of those studies. (Calais, et al., J Nucl Medicine, May 2018).
Results from a recently completed well designed prospective randomized trial (NCT03515577) further comparing the performance of the PSMA vs. Axumin PET/CT imaging in detecting the sites of recurrence are expected to be published soon. This is likely to confirm the findings from above the pilot comparison study.
PSMA IS NOT A SINGE AGENT
No PSMA PET agents are yet commercially available, with 68Ga-PSMA-11 under clinical trial investigation in multiple U.S. institutions (e.g. UCSF, UCLA, and Stanford University). “PSMA” is not one agent however. In addition to 68Ga-PSMA-11, there are many different PSMA-targeting agents in current development – somewhat of a dizzying array. Each has a different molecular configuration, requiring study to understand how well they work and how they are different from each other. Competition for the “best” PSMA agent is likely to be fierce. Below is a list of some of the agents currently under investigation:
68Ga-PSMA-11
68Ga-PSMA-617
68Ga-PSMA-I&T
68Ga-PSMA-R2
68Ga-PSMA-SR6
68Ga-NODAGA
68Ga-P16-093
64Cu-PSMA-617
64Cu-NODAGA
99mTc-MIP-1404
99mTc-HYNIC-PSMA
99mTc-J591
99mTc-EC0652
123I-MIP-1972
123I-MIP-1095
18F-DCFPyl
18F-DCFBC
18F-CTT1057
18F-PSMA-1007
A few limitations of PSMA-targeting agents are important to understand. Not all prostate cancers exhibit PSMA over-expression. In one study, about 8% of patients with prostate cancer did not show PSMA over-expression. Another study showed that there can be up to a 25% heterogeneity in expression in PSMA in an individual, thus in one person some of the cancer will be positive while other cancer deposits will be missed. Additionally, PSMA ligands are not completely specific for prostate cancer and several benign lesions such as thyroid adenoma, Paget’s disease, schwannoma, adrenal adenomas, and several types of vascular tumors (colon, breast, renal, liver, thyroid) may also exhibit increased PSMA expression. False positive celiac ganglia activity frequently has been noted in the upper abdomen.
Finally, most PSMA-targeting agents to date are significantly excreted in the urinary tract and urinary bladder. This is a huge issue as this obscures the prostate bed, making detection of locally recurrent lesions and lymph nodes in the lower pelvis challenging if not impossible to find – see the example below. When one considers that up to 30% of men with recurrence have cancer recurrence in this region, false negative PSMAs may have significant negative impact on their care. 18F-PSMA-1007 is one of the more recent additions to the long list of PSMA agents and is in the early pre-clinical study phase. This agent shows less prominent urinary excretion and may have a potential advantage compared to other PSMA agents in addressing this issue.
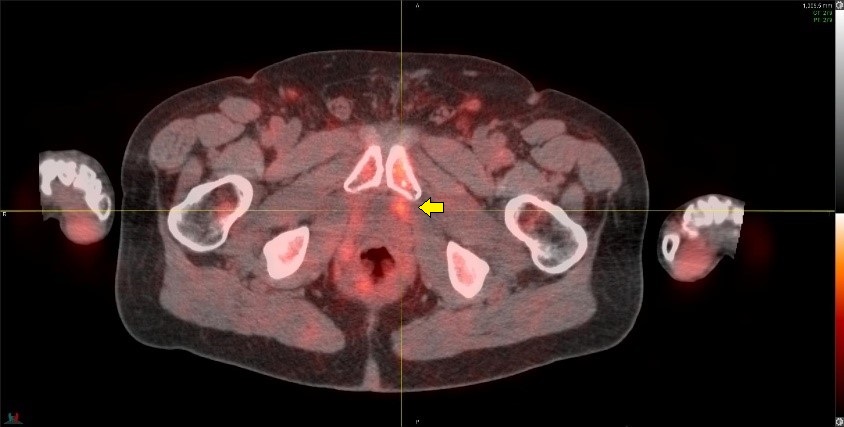
Ga68-PSMA-11 PET image in a patient with Gleason 4+5=9 disease, post prostatectomy and with a rising PSA of 1.1 ng/mL.
The PSMA study is negative. Prominent urinary bladder and ureteral tracer activity is present (arrows) which interfere with assessment of the prostate bed and pelvic lymph nodes. This study was performed after use of diuretics, which unfortunately was of little help in clearing the urinary activity.
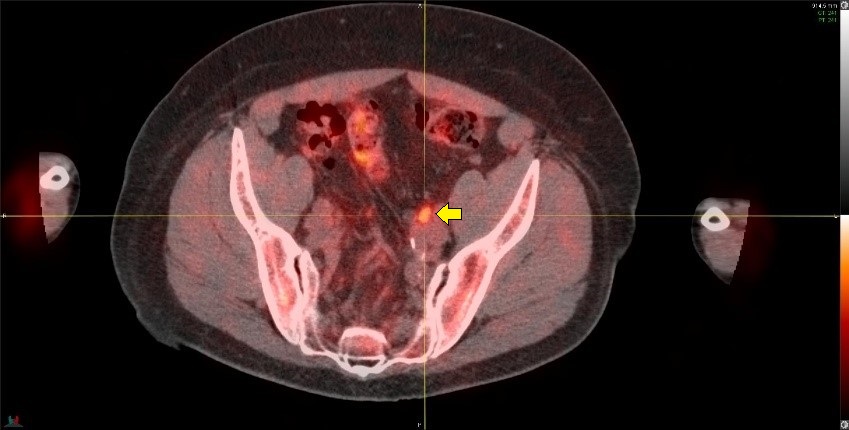
The image below shows a 11C-Acetate PET/CT imaging in the same patient as the above PSMA example showing axial, sagital and coronal views from left to right. A metabolic focus can be seen in the prostate bed at the anastomosis site (arrows). The lack of urinary tracer excretion of 11C-Acetate allowed visualization of a small lesion in this region, which was not apparent on PSMA imaging.
In Summary
Accurate re-staging is essential for optimal management decisions in recurrent prostate cancer. Current imaging with computerized tomography, magnetic resonance imaging, and 99Tc bone scanning lack the sensitivity to provide the needed information for accurate re-staging.
The excitement surrounding the current and emerging PET agents is appropriately exuberant. Radical breakthroughs are occurring in this area of imaging; however a fair amount of confusion exists, and further research is needed. There is still no “perfect” imaging methodology with 100% accuracy, with each of the current PET agents demonstrating some pros and cons.
The recent FDA approval of Auxmin is exciting as its 18F tag makes it more readily available than 11C tagged agents. Its overall performance, however, appears markedly suboptimal compared to other agents, particularly PSMA. The very high false-negative rate of Axumin scans in recurrent prostate cancer patients with relatively high serum PSA levels is concerning. Bone marrow and mostly muscle background activity appears to interfere with detection of lesions. The use of this agent perhaps should be avoided until further research is completed.
PSMA-targeted imaging appears to be promising and may provide higher sensitivity and specificity than other imaging agents to date. There are some important and practical limitations with PSMA however, as detailed above - lack of PSMA expression in some cancers, false positives in benign vascular lesions and inability to fully assess the lower pelvis due to high urinary excretion. Research is ongoing on PSMA agents to understand how well and accurately they perform.
Despite these limitations, PSMA-targeted imaging is clearly the future direction of most ongoing research. For patients with recurrent prostate cancer signaled by a rising PSA, performance of a sodium fluoride PET bone scan and seeking out one of the clinical trial studies for PSMA may be the best options at this time.
Fabio Almeida, MD is the Medical Director of Phoenix Molecular Imaging and Yuma Regional Cancer Center PET/CT providing his extensive clinical expertise in PET/CT imaging. He is an Integrative Oncologist and Nuclear Medicine Physician. His research is focused on applied medical informatics with emphasis on imaging and networking systems, optimization of fusion technology and volumetric tumor assessment for radiation therapy planning. He is one of the pioneers in the development and implementation of cross modality fusion for cancer imaging (SPECT, PET, CT and MRI) and PET/CT. He has authored and participated in several publications in radiology, oncology and information science. Dr. Almeida additionally provides extensive nutritional and lifestyle support through his Integrative Medicine Clinic.
P: 480.881.5621
www.drfabio.com
Updated 02/2019